How to Determine Limiting Reactant
Use the amount of limiting reactant for calculating the amount of product produced. Lastly if necessary calculate how much of the non-limiting agent is left in excess.

Chem 101 Dimensional Analysis Limiting Reagent Theoretical Yield Percent Yield Excess Reactant 2 Youtube
Get the number of moles of reacting substances from the given amounts of reactants.
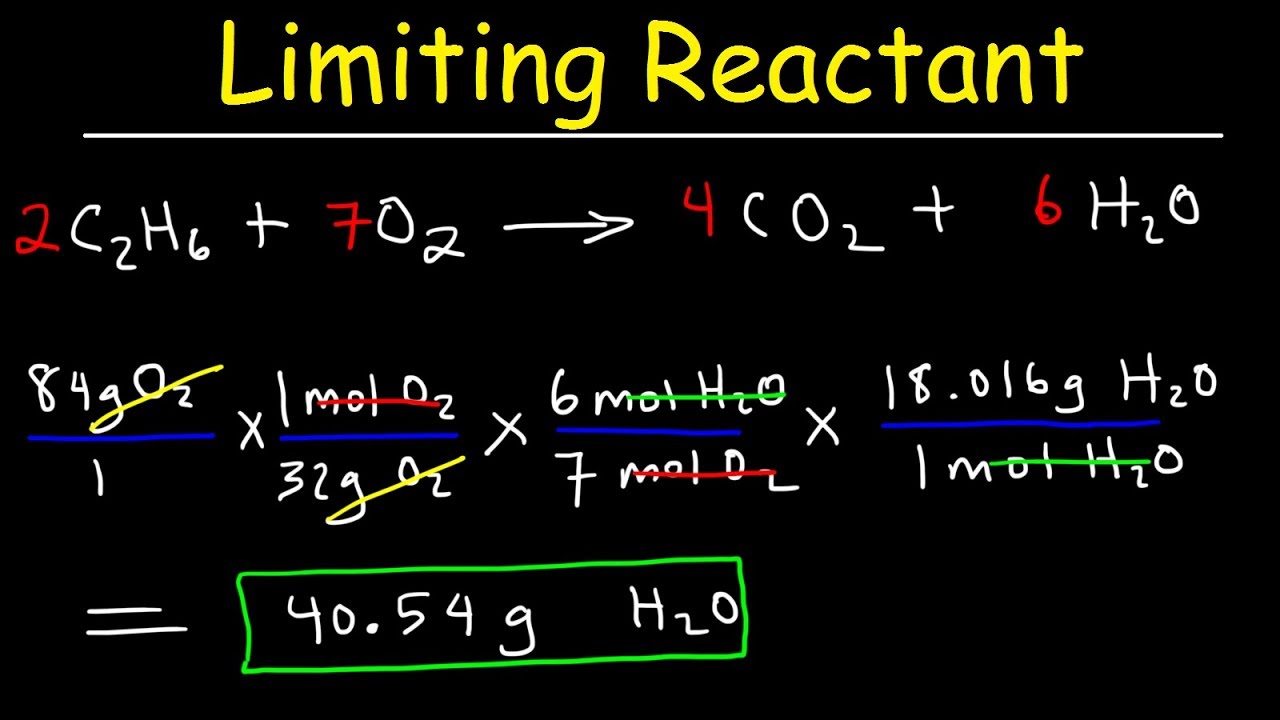
. Explains limiting reactant and how to determine which reactant in a chemical reaction is limiting. An example is shown below-. Answer if youre given the moles present of each reactant and asked to find the limiting reactant of a certain reaction then the simplest way to find.
Calculate the number of moles of. Divide by the coefficients of the hydrogen. Used to determine limitingexcess reagent.
To determine how much product Fe 3 O 4 will be made multiply the limiting reactant times the mole ratio of product to the limiting reactant and then multiply by the molar. We will work out a couple examples together and at the end of the. To determine the limiting reactant following steps should be taken.
If 25 ml of 0320 M barium chloride takes part in a reaction with. To determine which reactant is the limiting reactant first determine how much product would be formed by each reactant if all the reactant was consumed. Limiting reactant is also what prevents a reaction from continuing.
Click Create Assignment to assign this modality to your LMS. One way to determine the limiting reagent is to compare the mole ratio of the amount of reactants used. Yes the percentage of yield can be calculated from the concept of limiting reactant.
So in the above problem o 2 is the limiting reactant because limiting reactant reactant that produces least ml of product. Divide the actual number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. Determine the number of moles of each reactant.
A If the calculated MOLES NEEDED is greater than the MOLES HAVE for a given reactant then that reactant is the limiting reagent. The limiting reactant is the reagent compound or element to be totally consumed in a chemical reaction. We have a new and improved.
For the first method well determine the limiting reactant by comparing the. Follow us on Twitter Viking_Science. Calculate the number of moles H 2 given volume molar volume.
This method is most useful when. Lets go through the. This free limiting reactant calculator assists you to calculate limiting reactant that goes for finishing during the reaction and makes a limited amount of product.
All of the methods give the same answer though so you can choose whichever approach you prefer. Parrott teaches you how to determine the limiting reactant in a chemical reaction. Those are called the excess reactants.
How do you find the limiting reactant in a reaction. In this video we will learn how to determine the limiting reactant of a chemical reaction. Calculate the number of moles H 2 3022400.
2h2 o2 2h2o.

How To Find Limiting Reactant Quick Easy Examples Practice Problems Practice Questions Youtube

Limiting Reactant Practice Problems Youtube
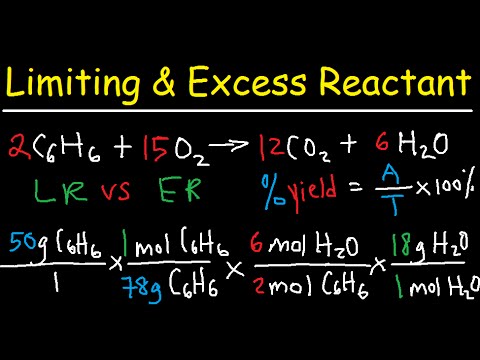
Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry Youtube

No comments for "How to Determine Limiting Reactant"
Post a Comment